Explain the Difference Between a Compound and an Element
If we look at the images closely image A represents a substance made up of the same kind of atoms. Air is made of atoms.

Difference Between Organic And Inorganic Compounds Definition Structure Properties Chemistry Lessons Chemistry Education Teaching Chemistry
Example - Hydrogen Carbon Oxygen.

. It is a pure substance. 6 rows The difference between an element and a compound is that an element is a substance made of. There are strong covalent bonds between atoms of the compound but in elements there can be metal bonds or weak non-covalent forces.
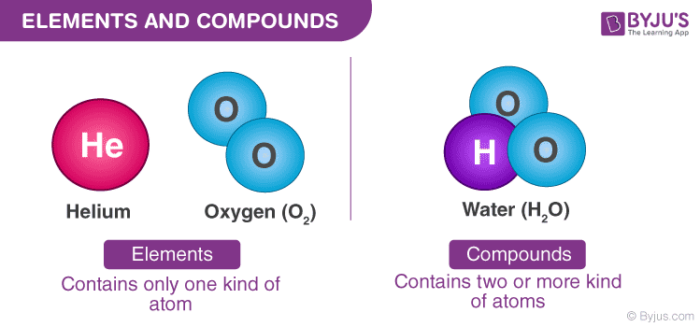
An element is a substance that is made entirely of one type of atom. Elements Compounds Mixtures. A mixture is made from two or more substances that are chemically different and are not chemically joined.
For example the element sodium is made up of only sodium atoms. They contain only one type of molecule. A molecule is a combination of two or more atoms.
Molecules that contain two or more different chemical elements are known as compounds. A compound contains atoms of different elements chemically combined together in a fixed ratio. In a compound elements are present in defined ratio.
The key difference between molecule of element and molecule of compound is that the molecule of element contains only one type of atoms whereas the molecule of compound contains two or more types of atoms. A substance that is made up of only one type of atom. The properties of an element often change completely when they are part of a compound.
An element is a substance made up of atoms of the same substance and has independent existence. It classifies into metals non-metals metalloids and noble gases. The isotopes of the same element differ in their atomic masses.
Compounds - It is a combination of two or more elements. If I had a green apple and a red apple that would be a compound. Everything is made of atoms.
An example of an element. Compare heterogeneous and homogeneous mixtures. This is why you cant begin to understand the difference between atoms and elements without first understanding what an atom is and what its.
Element- cant be broken down Mixture-more than one pure substance Compound- H2OH2O2. Explain the difference between an element and a compound. Every compound molecule or element you come across will be made of atoms.
While an example of compounds are Carbon dioxide CO 2 _2 2 Water H 2 _2 2 O Potassium chloride KCl Sodium chloride NaCl and etc. All compounds are molecules but not all molecules are compounds. Your computer made of atoms.
A compound is a molecule or group of two or more types of atoms. It is a pure substance. Elements are pure substances which are composed of only one.
An element is only composed of one type of atom while a compound can be composed of two or more different types of atoms. Compounds contain different elements in a fixed ratio arranged in a defined manner through chemical bonds. Elements are substances that are pure in their nature and are composed of.
Some of the best known examples are water H2O and sodium chloride or NaCl. The element is the chemical substance that is made up of similar kinds. For example humans are made of atoms.
Element - It is the basic unit of matter which cannot be broken down into substances by any way but can be combined to form new substances. He Helium H₂ and O₃. 8 rows Element Compound.
Example of elements are Hydrogen H Oxygen O Carbon C Helium He etc. A molecule is a substance that contains two or more atoms chemically joined such as H_2 O_2 A compound is a substance that is made up of two or more different elements that are chemically joined such as H_2O CO NaCl. Compounds Compounds are chemical substances made up of two or more elements that.
The main difference between element molecule and compound is that an element is a substance that cannot be further divided into parts by chemical means whereas a molecule is a substance that can be further divided into parts by chemical means and a compound is also a. Describe several techniques to separate mixtures. Each part in the mixture retains its own properties.
An element is a pure chemical substance made of same type of atom. In a compound two or more elements are chemically bound together. Elements Elements constitute the simplest chemical substances in which all the atoms are exactly the same.
We can classify molecules into different categories according to the number of atoms types of atoms. A compound is a substance formed when two or more elements chemically react with each other to form. Element compound pure substance mixture heterogeneous mixture homogeneous mixture.
All the balls are exactly of the same colour and type hence the answer. A substance that is made up of more than one type of atom bonded together. A combination of two or more elements or compounds which have not reacted to bond together.
Explain one difference between element and compound. 8 rows Question 1. An element is a simple substance that is made from one type of atom and cannot be broken down into simpler components by chemical or physical means.
On the other hand as its name implies the compounds are formed by different elements that are present in different proportions in the substance. Elements and compounds are the two forms in which pure substances exist. An element cannot be broken up by physical or chemical processes.

Worksheet For Elements And Compounds In Chemistry Google Search Elements Compounds And Mixtures Compounds And Mixtures Matter Science

Pin By Baris Karaman On Kimya Teaching Chemistry Science Lessons Teaching Science

Differences Between Elements Compounds Mixtures Compounds And Mixtures Elements Compounds And Mixtures Compounds Science

Pin By Kel Lala On Middle School Science Ideas Teaching Chemistry Chemistry Classroom Matter Science

Elements Mixtures And Compounds Vs Atoms And Molecules Compounds And Mixtures Elements Compounds And Mixtures Teaching Chemistry

Element Compound And Mixtures Match And Draw Compounds And Mixtures Elements Compounds And Mixtures Element

Elements Compounds And Mixtures Activity Cootie Catcher Foldable Review Game Elements Compounds And Mixtures Compounds And Mixtures Chemistry Classroom

Compound Vs Mixture Difference And Comparison Diffen Compounds Science Teaching Chemistry Chemistry Classroom

Atom Molecule Compound Mixture Teaching Chemistry Chemistry Education Elements Compounds And Mixtures

Elements Mixtures And Compounds Vs Atoms And Molecules School Chemistry Elements Compounds And Mixtures Compounds And Mixtures Chemistry Activities

Element Mixture Compound Activity Elements Compounds And Mixtures Heterogeneous Mixture Compounds And Mixtures

Matter Atoms Elements Molecules And Compounds Anchor Posters Atoms And Molecules For Kids Science Poster Science Display

Worksheet Elements And Compounds 1 Elements Compounds And Mixtures Compounds And Mixtures Matter Science

Difference Between Elements And Compounds Chemistry For Kids Mocomi Chemistry For Kids Compounds What Is An Element

Difference Between Compound And Mixture Definition Characteristics Types Of Bonding Compounds And Mixtures Study Chemistry Chemistry Classroom
Definition Of Compounds Elements Examples Types Classification With Videos

Week 2 Chapter 4 Atoms Molecules And Ions Teaching Chemistry Chemistry Classroom Chemistry Education

Differences Between Elements Compounds Mixtures Compounds And Mixtures Elements Compounds And Mixtures Compounds Science

Elements Compounds And Mixture Anchor Chart Science Anchor Charts Science Chart 6th Grade Science
Comments
Post a Comment